--------------------------------
Indications and Dosage
Inhalation/Respiratory
Acute bronchospasm
Adult: Treatment and prevention in patient with reversible obstructive airway disease: As metered-dose aerosol or dry powder inhaler (90 or 100 mcg/actuation): 1 or 2 inhalations every 4-6 hours. Max: 800 mcg daily.
Child: As metered-dose aerosol or dry powder inhaler: 4-11 years 1 or 2 inhalations; ≥12 years Same as adult dose.
Inhalation/Respiratory
Prophylaxis of exercise-induced bronchospasm
Adult: As metered-dose aerosol or dry powder inhaler (90 or 100 mcg/actuation): 2 inhalations 15-30 minutes prior to exercise.
Child: As metered-dose aerosol or dry powder inhaler (90 or 100 mcg/actuation): 4-11 years 1 or 2 inhalations 15-30 minutes prior to exercise; ≥12 years Same as adult dose.
Inhalation/Respiratory
Chronic bronchospasm
Adult: Via nebuliser: 2.5-5 mg up to 3-4 times daily.
Child: ≥4 years Same as adult dose.
Oral
Bronchospasm
Adult: Treatment and prevention in patient with reversible obstructive airway disease: As syr/immediate-release tab: 2-4 mg 3-4 times daily, may be increased up to max 8 mg 3-4 times daily as tolerated. As extended-release tab: 4-8 mg 12 hourly.
Elderly: As syr/immediate-release tab: Initially, 2 mg 3 or 4 times daily.
Child: As syr/immediate-release tab: 2-6 years 1-2 mg; >6-12 years 2 mg; >12 years Same as adult dose. All doses are given 3 or 4 times daily. As extended-release tab: 6-12 years 4 mg 12 hourly.
Adverse Reactions
Significant: Hypersensitivity reactions (e.g. urticaria, angioedema, rash, bronchospasm, oropharyngeal oedema), hypokalaemia (high doses).
Cardiac disorders: Tachycardia, palpitations, chest pain. Rarely, cardiac arrythmias (e.g. atrial fibrillation, supraventricular tachycardia, extrasystoles), myocardial ischaemia.
Gastrointestinal disorders: Nausea, vomiting, mouth and throat irritation (inhalation).
General disorders and administration site conditions: Weakness.
Metabolism and nutrition disorders: Hyperglycaemia. Rarely, lactic acidosis (high doses).
Musculoskeletal and connective tissue disorders: Muscle cramps.
Nervous system disorders: Tremor, headache, dizziness, drowsiness, restlessness.
Psychiatric disorders: Nervousness, irritability, insomnia.
Respiratory, thoracic and mediastinal disorders: Pharyngitis, rhinitis, pulmonary oedema (parenteral).
Vascular disorders: Peripheral vasodilation, flushing.
Potentially Fatal:
Rarely, paradoxical bronchospasm.
Pregnancy Category (US FDA)
Inhalation/Respiratory/IV/Parenteral/PO: C
Monitoring Parameters
Monitor FEV1 peak flow, and/or other pulmonary function tests; blood pressure, heart rate, CNS stimulation; serum glucose, K and creatinine; asthma symptoms; arterial or capillary blood gases if needed; lactate, and ECG (IV).
Overdosage
Symptoms: Tachycardia, tremor, hyperactivity, metabolic effects (e.g. hypokalaemia, lactic acidosis), headache, nervousness, dry mouth, palpitations, dizziness, fatigue, malaise, insomnia, arrhythmias, anginal pain, seizures. Children may experience nausea, vomiting, hyperglycaemia when taken orally. Management: Symptomatic treatment. Monitor serum K and lactate levels. May give cardioselective β-blocker with caution. May give oral or IV potassium for hypokalaemia as needed.
Drug Interactions
Increased risk of hypokalaemia with corticosteroids, diuretics (e.g. loop, thiazide) and xanthines (e.g. theophylline). Increased vascular effects with MAOIs, tricyclic antidepressants (TCAs). May cause severe bronchospasm when used with β-blockers (e.g. propranolol). May decrease serum concentrations of digoxin.
Action
Description:
Mechanism of Action:
Salbutamol is a direct-acting sympathomimetic that acts on β2-receptors to relax bronchial smooth muscle with less prominent effect on the heart. It activates adenyl cyclase, the enzyme that stimulates the production of cyclic adenosine-3', 5'-monophosphate (cAMP). Increased cAMP leads to activation of protein kinase A, which inhibits phosphorylation of myosin and lowers intracellular ionic Ca concentrations, resulting in muscle relaxation.
Synonym: albuterol
Onset: ≤5 minutes (inhalation); 5.4-8.2 minutes (oral inhalation); ≤30 minutes (oral).
Duration: Inhalation: 3-6 hours. Oral inhalation: Approx 2-6 hours. Oral: 6-8 hours (immediate-release); up to 12 hours (extended-release).
Pharmacokinetics:
Absorption: Readily absorbed from the gastrointestinal tract. Time to peak plasma concentration: Inhalation: 30 minutes. Oral inhalation: 25-30 minutes. Oral: ≤2 hours (immediate-release); 6 hours (extended release). Bioavailability: Approx 50%.
Distribution: Crosses the placenta. Plasma protein binding: 10%.
Metabolism: Metabolised in the liver to an inactive sulfate conjugate.
Excretion: Mainly via urine (80-100% [oral inhalation]; 76% over 3 days, [60% as metabolite; oral]); faeces (<20% [oral inhalation]; 4% [oral]). Elimination half-life: Oral inhalation: 3.8 to approx 5 hours. Oral: 5-6 hours (immediate-release); 9.3 hours (extended-release). IV: 4-6 hours.
Chemical Structure
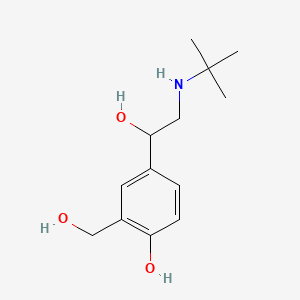 Salbutamol
Salbutamol
Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 2083, Salbutamol. https://pubchem.ncbi.nlm.nih.gov/compound/Salbutamol. Accessed Oct. 24, 2023.
Storage
Dry powder or metered dose inhalers/tab/syr: Store below 25°C. Solution for inj/nebule: Store below 30°C. Protect from light.
MIMS Class
Antiasthmatic & COPD Preparations / Drugs Acting on the Uterus



